

Braunwald, EugeneĮuropean Journal of Heart Failure (2016) 18, 1153–1161 RESEARCH ARTICLE doi:10.1002/ejhf.595 Efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation and heart failure: insights from ENGAGE AF-TIMI 48 1 1 1 1 Giulia Magnani, Robert P. Nordio, Francesco Metra, Marco Moccetti, Tiziano Mitrovic, Veselin Shi, Minggao Mercuri, Michele Antman, Elliott M. No additional events occurred in patients who had multiple cardioversions while on study drug (267 additional cardioversions) in the 30 days post–repeat cardioversions.Efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation and heart failure: insights from ENGAGE AF‐TIMI 48 Efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation and.
#Engage af timi 48 trial
From cardioversion to the end of the trial (median follow‐up 25 months), there were no statistically significant differences in primary efficacy and safety endpoints between treatment groups.

There were no major bleeding events and 1 death (higher‐dose edoxaban) in the 30 days after cardioversion.
In the 30 days after cardioversion, stroke or SEE occurred in 2 patients on the lower‐dose edoxaban regimen none occurred with warfarin or higher‐dose edoxaban (Table (Table1). In the warfarin group, the median time between the last INR measurement and cardioversion was 6 days (IQR, 1–13 days) and median INR value was 2.5 (IQR, 2.1–3.0). There were no differences between randomized treatment groups in baseline characteristics among these 365 patients. Compared with others in the trial, the 365 patients undergoing a first cardioversion while on study drug were, at the time of randomization, significantly younger (median age, 70 vs 72 years) and more likely to have paroxysmal AF (47% vs 25%), a CHADS 2 score ≤3 (88% vs 77%), and to have been enrolled in North America (41% vs 22% P < 0.001 for each). There were a total of 632 electrical cardioversion attempts performed while on study drug in 365 patients occurring on average 348 days (interquartile range, 86–526 days) after randomization. We excluded 200 cardioversions that occurred >3 days after the most recent dose of blinded study anticoagulant (edoxaban or warfarin ie, during a period of interruption of blinded study drug of ≥4 days).

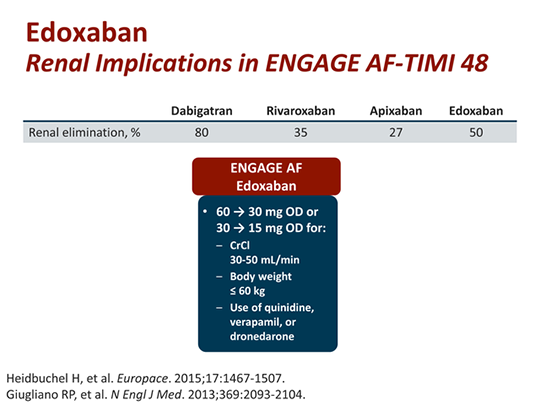
There were 832 attempted electrical cardioversions performed to restore sinus rhythm in patients with AF or atrial flutter (we considered all shocks delivered on the same date as 1 cardioversion attempt) during the trial. Both edoxaban regimens were noninferior to warfarin in preventing the composite of stroke (including both hemorrhagic and nonhemorrhagic stroke) or systemic embolic events (SEE) while significantly reducing major bleeding, intracerebral hemorrhage, and cardiovascular mortality. Major exclusion criteria were AF due to reversible cause, severe renal impairment, increased bleeding risk, mechanical heart valve, or moderate to severe mitral stenosis. Patients with moderate renal dysfunction, weight ≤60 kg, or concomitant use of P‐glycoprotein inhibitors received a 50% dose reduction of edoxaban (or matching edoxaban‐placebo). This 3‐arm, randomized, double‐blind, double‐dummy, global phase 3 clinical trial compared a once‐daily higher‐dose edoxaban (60 mg/30 mg) regimen, a lower‐dose edoxaban (30 mg/15 mg) regimen, and warfarin (target international normalized ratio of 2.0–3.0) in 21 105 patients with AF and CHADS 2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke/transient ischemic attack/thromboembolism) score ≥2.


 0 kommentar(er)
0 kommentar(er)
